SOLVED: Students working in lab accidentally spilled 13 Lof 3.0 MHzSO4 solution: They find a large container of acid neutralizer that contains baking soda, NaHCOz: How many grams of baking soda will

SOLVED: Some sulfuric acid is spilled on a lab bench. You can neutralize the acid by sprinkling sodium bicarbonate on it and then mopping up the resultant solution. The sodium bicarbonate reacts
What's the best ratio for a baking soda/citric acid reaction? What does the reaction formula look like? - Quora
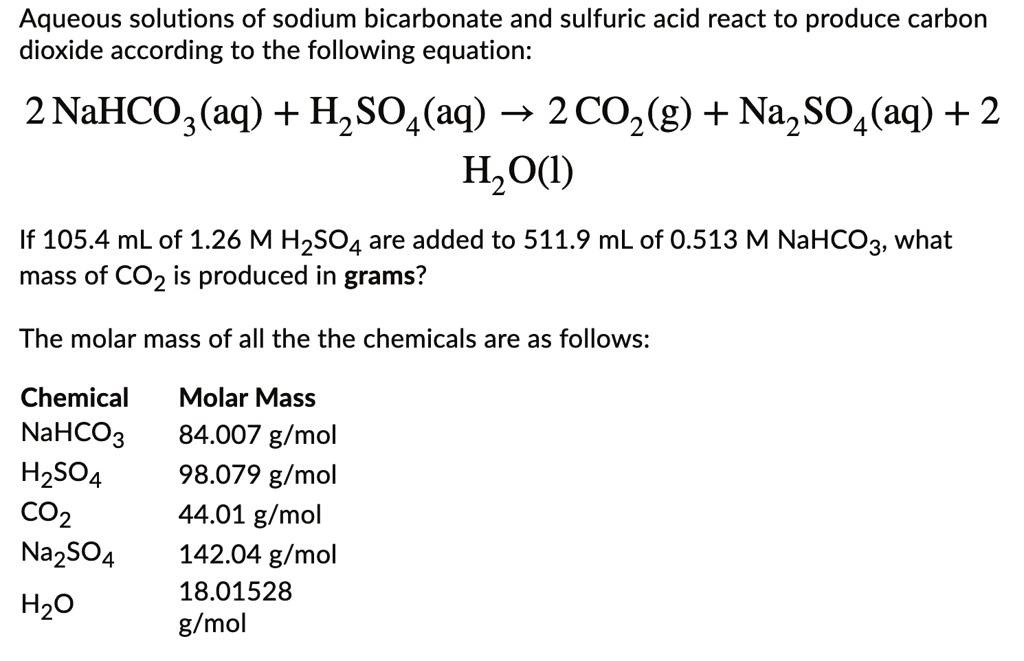
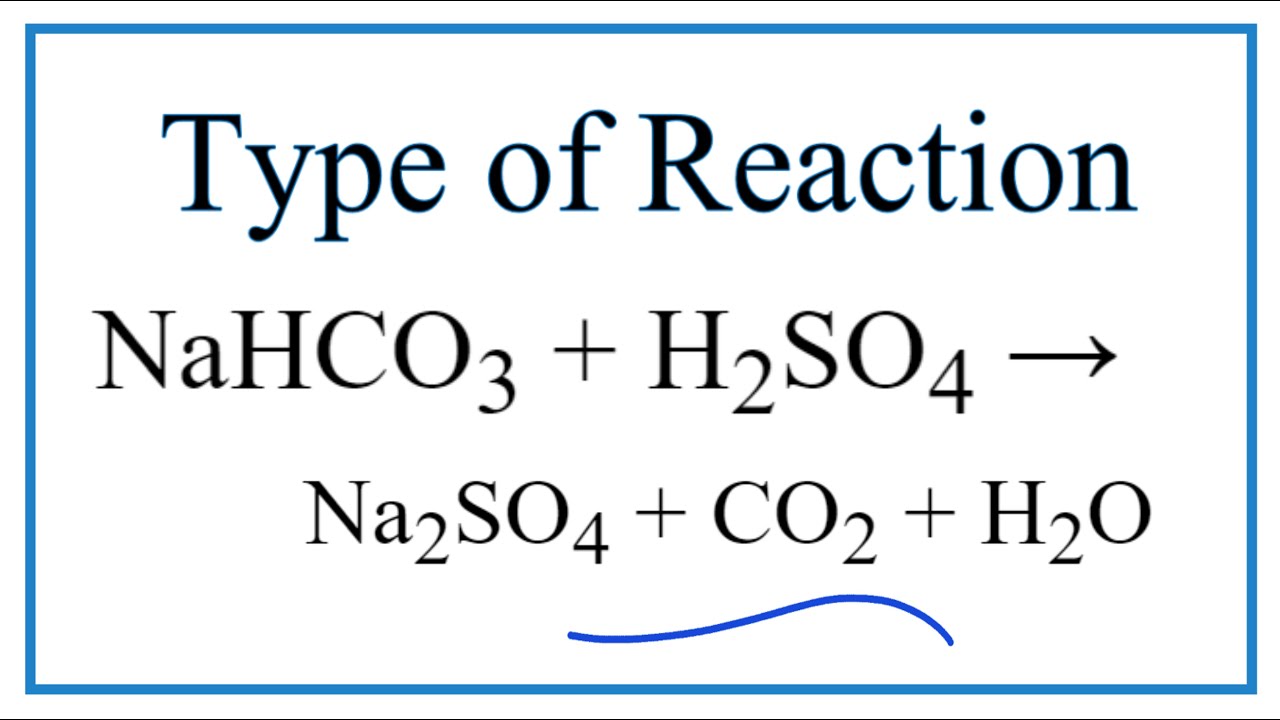
SOLVED: Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: 2 NaHCOs(aq) + H,SO4(aq) 3 2CO2(g) + NazSO4(aq) + 2 HzO() If 105.4